
Brassica
PRS – (Protein from Seed)
16 % v/v glycerol
Bring 200 grams (= 160 ml) of glycerol (q.c.w. Sigma G-7757) and 800 ml distilled water in a beaker. Mix well and bring to a final volume of 1000 ml with distilled water. Store in the refrigerator.
Sepalyte pH 3-10 (Cat.No. 42008 ProTec Bioseparation)
Ready to hand Sepalyte solutions. Store in refrigerator
Acrylamide/ bis acrylamide 29 : 1 solution
Ready to hand acrylamide solution (q.c.w. Sigma A-3574). Store in refrigerator.
0.1N Sodium hydroxide
4.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 1000 ml distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature
0,1% w/v riboflavin
0.05 g riboflavin (q.c.w. Sigma R-0508) in 50 ml 0.1 N sodium hydroxide.
Store in the refrigerator for up to 2 weeks.
10% w/v ammonium persulfate
0.5 g ammonium persulfate (q.c.w. Sigma A- 3678) in 5.0 ml distilled water.
Store in refrigerator for one week
4 N sodium hydroxide
16.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 100 ml distilled water. Store in the refrigerator.
PRS extraction solution
2.5 ml PH 3-10 (Cat. No.42008 ProTec Bioseparation) in 500 ml distilled water. Add a trace (tip of a small spatula) of Orange G (q.c.w. Sigma O-1625). Store in refrigerator.
Cathode fluid 10
Bring 0.90 g L-arginine base (Sigma A- 5006)+ 0.70 g L-lysine (Sigma L-5501)+ 24.0 ml ethylene diamine (q.c.w. Sigma E-9890) in 200 ml water. Store in refrigerator.
Anode fluid 3
Bring 0.68 g L-glutamic acid (q.c.w. Sigma G-6904) and 0.72 L-aspartic acid (q.c.w. Sigma A-8949) in 200 ml distilled water. Stir until fully dissolved. Store in refrigerator.
Trichloroacetic acid( TCA) 20 % w/v
Bring 400 g of trichloroacetic acid (q.c.w. Sigma T-4885) and 1500 ml water in a beaker. Stir until fully dissolved. Bring to a final volume of 2000 ml with distilled water. Store at room temperature.
Methanol acetic acid distilled water (MAD) stock solution
Bring together 660-ml methanol 100% (q.c.w. BDH 29192) and 340 ml acetic acid 100% (q.c.w. BDH 27013) Add 2 x small spatula tips of dithiotreitol (Sigma D-9163). Store at room temperature.
MAD working solution
Bring together 200 ml stock MAD and 800 ml water. Mix well before use. This solution is reusable, when used on the same day of making. Do not store.
Potassium dichromate 0.1 w/v %
300 mg potassium dichromate (q.c.w. Sigma P-6435) in 300 ml distilled water. Add 3 drops of nitric acid 65 -70 % (q.c.w. BDH 29335). Stir until fully dissolved. This solution is reusable when used on the same day of making. Keep the solution in the dark. Do not store.
Silver nitrate 0. 2 w/v %
1.0 g silver nitrate (q.c.w. Sigma S-0139) in 500 ml distilled water. Do not store.
Sodium carbonate 15 w/v % Stock solution
Mix 100-ml stock sodium carbonate 15 w/v% and 400 ml distilled water. Add just after the silver nitrate step in the staining procedure 2 ml formaldehyde 37 % (q.c.w. Sigma F-1268).
Acetic acid 0.1 v/v %
Mix 10-ml acetic acid 100 % (q.c.w. BDH 27013) with 1000 ml distilled water.
TEMED
Ready to use TEMED Sigma ( T-9281).
Gel preparation
9.5 ml 16 % glycerol
2.0 ml Acrylamide/bis 29:1solution
1.0 ml Sepalyte pH 3-10
0.065 ml 0.1 % w/v riboflavin
0.012 ml TEMED
0.035 ml 10% ammonium persulfate
Add the above reagents and swirl to mix. Pour the gel according to the flap technique and allow polymerizing for at least 4 hours under light. Store the gels in a sealed bag in the refrigerator for up to 2 weeks.
Sample preparation
Single seeds are placed into a 96 well micro plate. To each well, an aliquot * of 0.5 % Sepalytees 3-10 is added. The seeds are homogenized using the Terminator for 3 minutes and centrifuged for 10 minutes at 3000 rpm. at 10° C (or at room temperature).
fraction size 1.25-1.49 mm + 150 ml 0.5 % Sepalyte 3-10
fraction size 1.50-1.74 mm + 175 ml 0.5 % Sepalyte 3-10
fraction size 1.75-1.99 mm + 200 ml 0.5 % Sepalyte 3-10
fraction size 2.00-2.24 mm + 225 ml 0.5 % Sepalyte 3-10
fraction size 2.25-2.50 mm + 250 ml 0.5 % Sepalyte 3-10
Electrophoresis
Turn the cooling supply on and set at a temperature of ± 15° C. Remove the gel from the glass plates. Clean the back of the gel with methanol/ethanol. Place the gel onto the cooling plate with several ml of water. The gel can be divided into two or three parts. Space the electrodes evenly across the gel, alternating cathode (black electrode) and anode (red electrode). Place the electrode plateau directly onto the gel, making electrode imprints in the gel. Blotting paper wicks (1 x 6-x 260 mm) are used. Wet the cathode wicks with cathode fluid 10 and the anode wicks with anode fluid 3. Gently blot the wicks but keep them fairly wet. Place the cathode wicks onto the cathode imprints in the gel and the anode wicks onto the anode imprint. Bring the electrode plateau on top of the electrode wicks.
Power settings are for one gel (double the mA and Watts when running two gels).
Power settings for gels divided into 2 parts are also provided below.
Prefocusing
Run 1: 400 V–30 mA–5 W–75 V/h
(2 parts) 600 V-60mA-12W-75V/h
Sample application
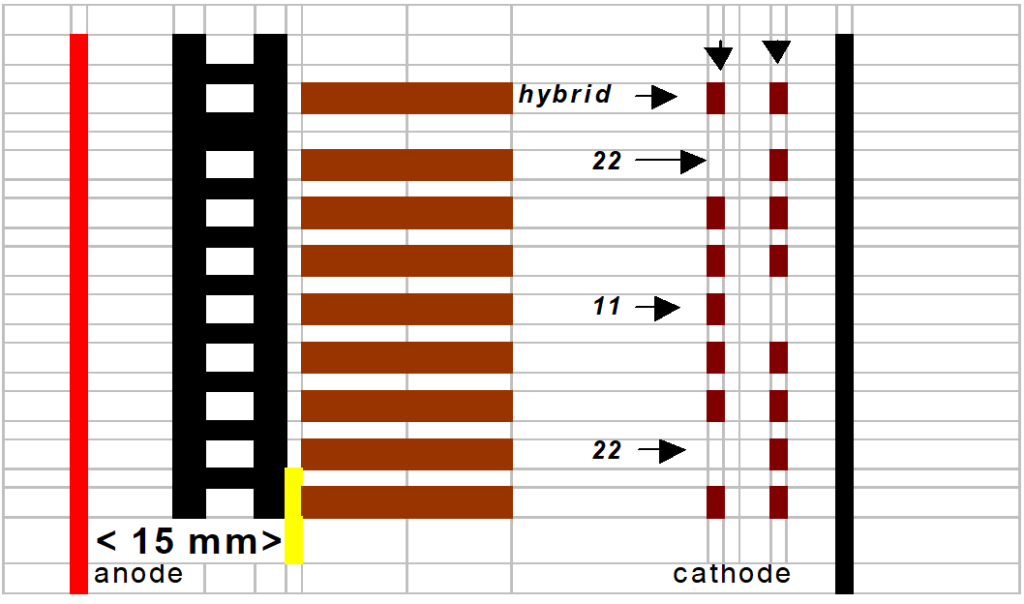
After the prefocusing step, the 96 templates are positioned ± 15 mm from the anode (red electrode). See cartoon image section “gel interpretation 9.1.6.“
Each sample well is filled with 8 µl of supernatant.
Focusing
Run 2: 200 V–30 mA–5 W–50 V/h
(2 parts) 200V-60mA-12 W-50V/h
Run 3: 600 V–30 mA–5 W–500 V/h
(2 parts) 1000V-60mA-12W-1000 V/h
After the gel has finished running, remove the gel from the cooling plate and place into an appropriate staining tray.
Merill silver staining
Pour on the gel ±300-ml of 20% TCA solution. Let the proteins precipitate for 5 minutes.
After the 5 minutes of fixation, swirl the tray for 15 minutes.
Rinse off the excess of TCA.
Three washing steps:
Pour on the gel ± 300-ml MAD working solution and swirl for 5 minutes.
Strain off the MAD or reuse this solution for the next washing step 1.
Again pour on the gel ± 300-ml MAD working solution and swirl for 5 minutes.
Strain off the MAD or reuse this solution for the next washing step 2.
Again pour on the gel ± 300-ml MAD working solution and swirl for 5 minutes.
Strain off the MAD or reuse this solution for the next washing step 3.
Submerge the gel in ± 300-ml potassium dichromate 0.1 w/v % and swirl for 5 minutes.
Discard or reuse the potassium dichromate after this step.
Submerge the gel in ± 300-ml silver nitrate 0.2 w/v % and swirl for at least 20 minutes.
Discard the silver nitrate after this step. Do not reuse the silver nitrate solution.
The first TWO submerging steps (± 100 ml) with the sodium carbonate working solution should be done swiftly (10 sec.) to prevent chloride precipitation onto the gel. The THIRD one
(± 200 ml) should be monitored until the stain is appropriate and STOP IMMEDIATELY with a 0.1% acetic acid solution. Discard the sodium carbonate solution after each step.
Rinse the gel with distilled water. The gel is air-dried.
Gel interpretation
The nearest band of interest from the anode is genotyped “11”.
The farthest band of interest from the anode is genotyped “ 22”.
The hybrid is genotyped “1/2” in case that the female is 11 and the male 22.
The hybrid is genotyped “2/1” in case that the female is 22 and the male 11.
ProTec Bioseparation (Protein Electrophoresis)
